LSU Pediatrics and Children's Hospital Play a Major Role in New SCID Treatment Research Protocol
LSU Pediatrics Hematology Oncology/HSCT and Allergy Immunology faculty members are
part of the Primary Immunodeficiency Treatment Consortium (PIDTC), which is currently
investigating a new research protocol, Conditioning SCID Infants Diagnosed Early (C-SIDE),
for infants with specific forms of severe combined immune deficiency (SCID). This
is an inherited condition in which the body is unable to fight off serious and life-threatening
infections. Babies with SCID are at risk for many infections and rarely live past
infancy without treatment. The curative treatment for SCID is stem cell transplant
(SCT).
C-SIDE seeks to evaluate if babies with SCID can achieve good immunity with less short
and long-term risks of complications after SCT related to high doses of chemotherapy
and long-term immunosuppression. There are two interventions being investigated to
answer this important question.
First, patients are randomized to receive a standard or lower dose of busulfan as
conditioning prior to SCT. Lower doses of busulfan result in lower risk of immediate
post-transplant complications such as mucositis and veno-occlusive disease of the
liver. Late effects of higher doses of busulfan include lung disease, infertility,
and secondary malignancies.
Second, a technique of ex-vivo T cell reduction is utilized for all patients wherein
harmful T cells are removed from the stem cell product prior to infusion. Specifically,
alpha-beta-T cells, which recognize MHC and may mediate graft versus host disease
(GVHD), are depleted from the donor specimen while gamma-delta-T cells which are intrinsically
blind to MHC remain in the donor specimen. This technique allows for these immunodeficient
patients to be followed in the post transplantation period without systemic immunosuppression.
Reduction in chemotherapy doses and avoidance of post-transplant immunosuppression
are predicted to reduce the morbidity and mortality of SCT.
The C-SIDE research protocol is available only to infants who do not have a fully
HLA-matched sibling donor (MSD) available. It is well established that a fully HLA-matched
sibling donor offers the best outcome to SCT. This type of match has less risk for
complications such as graft rejection or GVHD. Infants with SCID who have an MSD often
need very little, if any, pre-transplant chemotherapy, depending on the specific form
of SCID. Therefore, the C-SIDE protocol seeks to build evidence for the best protocol
when there is no MSD available. Donors used for transplant in the C-SIDE study must
be unrelated or half-matched related (haploidentical) donors.
Numerous outcomes related to transplant will be evaluated. These include but are not
limited to stem cell engraftment, development of GVHD, donor chimerism, and overall
survival. The pediatrics hematology oncology/HSCT and allergy immunology team remains
in constant contact with the national study committee, and the office of clinical
trials here at LSU Health Sciences Center and Children's Hospital have been invaluable
in this time intensive process. Approximately 9-18 months after the transplant, vaccinations
will be administered, and vaccine titers will be measured. The primary goal for any
patient with SCID is the development of a new immune system capable of protecting
the patient from new infections and an appropriate response to vaccinations.
Dr. Lolie Yu leads the pediatric SCT team and is the principal investigator for the
LSU Health New Orleans/Children's Hospital study site. She and Dr. Zachary LeBlanc
(also pediatric SCT) were instrumental in bringing the C-SIDE protocol to Children's.
“The LSU/CHNOLA HSCT program established in 1990 is very proud that we are part of
the PIDTC and we are able to offer this innovative treatment to these very rare and
complex patients as well to all other children in Louisiana without the need to go
out of state for their care. It is the only credentialed and FACT approved Pediatric
HSCT in the state,” Dr. Yu said.
Dr. LeBlanc added, “Treatment of SCID requires a multidisciplinary team of medical
professionals. We are privileged to have Louisiana's only such team capable of caring
for these infants at Children's Hospital. We believe our core mission is caring for
the most vulnerable patients with the best treatments available to any physician in
the world. Being a part of the PIDTC and enrolling patients on C-SIDE is only one
such example of the execution of this mission. We are thankful to our brave patients
and their families for enrolling in clinical trials.”
 Dr. Luke Wall, a clinical immunologist who is also part of the PIDTC, and the Pediatric
Allergy Immunology Division take the lead role in elucidating the specific SCID genotype
and immunologic phenotype, and management of the infants leading up to the time of
transplant induction.
Dr. Luke Wall, a clinical immunologist who is also part of the PIDTC, and the Pediatric
Allergy Immunology Division take the lead role in elucidating the specific SCID genotype
and immunologic phenotype, and management of the infants leading up to the time of
transplant induction.
“While Louisiana was among the first states to begin a brief pilot SCID newborn screening
program nearly a decade ago, the screening test for SCID was just implemented permanently
in Louisiana on December 10, 2018. This now allows us, as clinical immunologists,
to diagnose SCID early, hopefully prior to the infant's first infection,” Dr. Wall
said. “Previously, infants with SCID came to recognition only after experiencing opportunistic
or life-threatening infections and sadly, some of these were too ill for transplant.
Considering that we now have statewide screening, we are delighted to be one of the
first C-SIDE study sites to enroll infants, nationally. In the world of immune deficiencies
and transplant immunology, we have much to learn. We are so proud to be part of this
national study which will hopefully yield results which shape clinical guidelines
and improve outcomes.”
“While newborn screening now allows the advantage of early diagnosis of SCID, the
clinical, psychological, and immunological nuances surrounding the diagnosis remain
exceedingly complex. This is the most profound condition we treat as immunologists.
Infants almost never follow the textbook, and it is not unusual to get conflicting
immunologic lab patterns which require repeated and deeper laboratory investigation.
All the while, the emotional and psychological impact on the family is profound. Now
that we are identifying these infants early, we have to tell brand new parents of
a vibrant, healthy-looking infant that their baby has a life-threatening condition
and must undergo an intense treatment, such as stem cell transplant. This requires
many players in a multidisciplinary team, including our genetic counselor, social
services, and often psychology,” Dr. Wall added.
 PIDTC is a collaboration of 41 North American centers studying therapy for rare primary
immune deficiency diseases (PIDs), including severe combined immune deficiency (SCID),
Wiskott-Aldrich syndrome (WAS), and chronic granulomatous disease (CGD). An additional
three European centers have partnered with the PIDTC to study CGD. Natural history
protocols of the PIDTC analyze outcomes of treatment for rare PIDs in multicenter
longitudinal, retrospective, prospective, and cross-sectional studies. Since 2009,
participating centers have enrolled more than 800 subjects on PIDTC protocols for
SCID, and enrollment in the studies on WAS and CGD is underway.
PIDTC is a collaboration of 41 North American centers studying therapy for rare primary
immune deficiency diseases (PIDs), including severe combined immune deficiency (SCID),
Wiskott-Aldrich syndrome (WAS), and chronic granulomatous disease (CGD). An additional
three European centers have partnered with the PIDTC to study CGD. Natural history
protocols of the PIDTC analyze outcomes of treatment for rare PIDs in multicenter
longitudinal, retrospective, prospective, and cross-sectional studies. Since 2009,
participating centers have enrolled more than 800 subjects on PIDTC protocols for
SCID, and enrollment in the studies on WAS and CGD is underway.
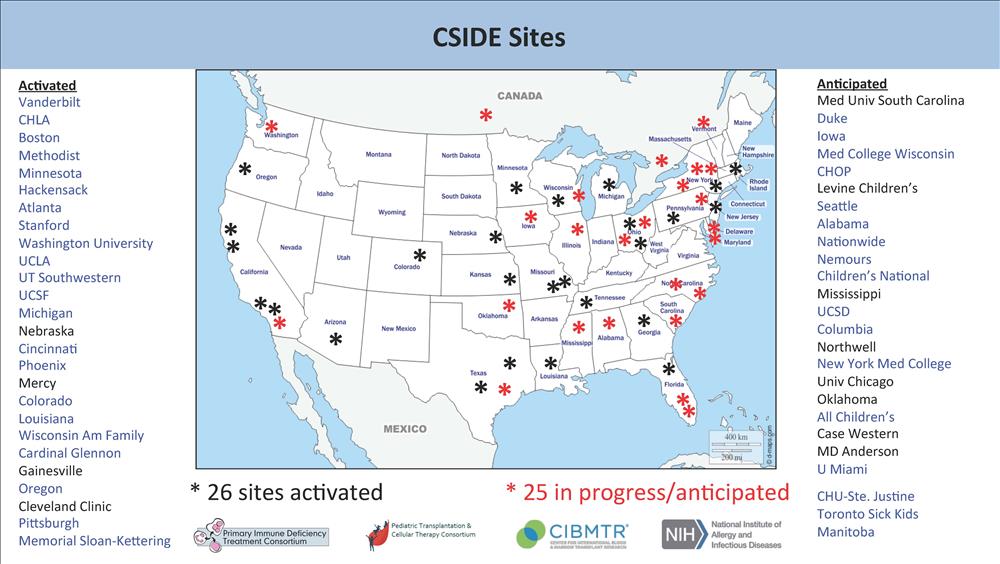
The PIDTC 10th Annual Scientific Workshop was held virtually on April 29, 2020. A
highlight of the meeting was an update on the new C-SIDE research protocol. LSU Pediatrics/Children's
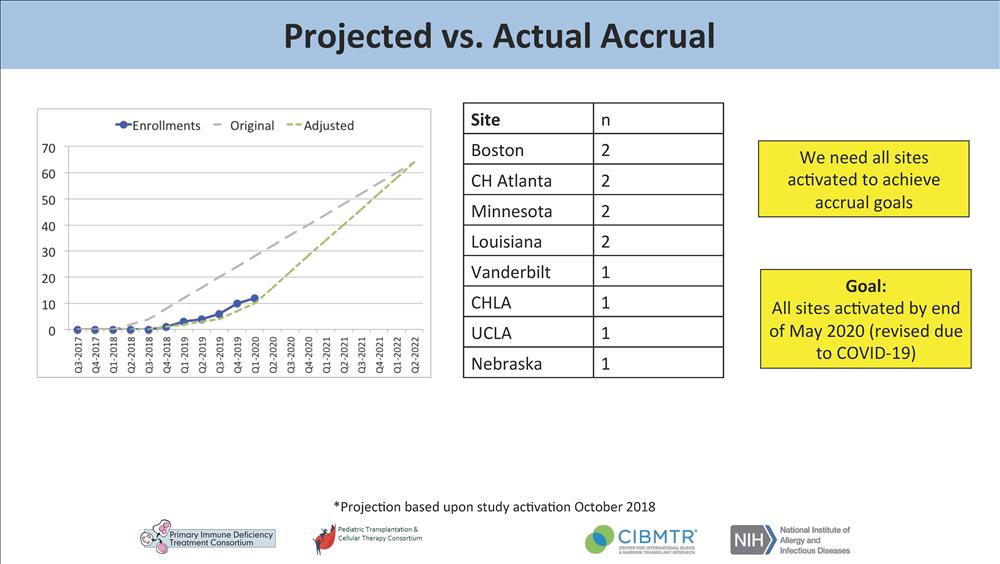
Hospital is one of only 26 sites currently activated to enroll patients. Remarkably,
our institution has enrolled two out of only 12 infants accrued nationally to date
(only eight centers have accrued subjects to date).
Congratulations to the hematopoietic stem cell transplant team, the immunology team,
clinical and research nurses and the Clinical Trials Center (CTC) at Children's Hospital
in the collaborative care and research surrounding these complex infants.
