Introduction to Acid-Base Physiology
I. Definitions
A. Acid - can donate a hydrogen ion
B. Base - can accept a hydrogen ion
C. Strong acid - completely or almost completely dissociates into a hydrogen ion and its conjugate base in aqueous solution; a weak acid is only slightly ionized in aqueous solution. A strong acid usually has a weak conjugate base and a weak acid usually has a strong conjugate base. Do not confuse the strength of an acid or base with its concentration.
D. Buffer - mixture of substances in aqueous solution, usually a combination of a weak acid and its conjugate base, that can resist changes in [H+] when strong acids or bases are added.
II. Quantification of Acidity
A. pH = -log [H+]
B. pH of 7.10 is [H+] of 80 nM/L
C. pH of 7.30 is [H+] of 50 nM/L
pH of 7.40 is [H+] of 40 nM/L
pH of 7.50 is [H+] of 32 nM/L
pH of 7.70 is [H+] of 20 nM/L
D. Note that as [H+] increases, pH decreases. An increase of 0.3 pH units indicates that [H+] is cut in half; a decrease of 0.3 pH units indicates that [H+] is doubled.
E. By convention:
1. Arterial pH< 7.35 is acidemia
2. Arterial pH >7.45 is alkalemia
3. Acidosis and alkalosis are underlying disorders
III. Sources of Acids in the Body
A. CO2 + H2O ![]() H2CO3 ( a volatile acid)
H2CO3 ( a volatile acid)
Lungs remove about 15,000 - 25,000 mmol of carbon dioxide per day.
B. Fixed acids produced as a result of metabolism. About 70 mEq of H+'s is removed mainly by the kidneys (a minor portion is removed by the G.I. tract) each day. Fixed acids normally represent only about 0.2% of the total body acid production. May be much higher - e.g. in diabetic ketoacidosis.
IV. Buffer systems of the human body - Isohydric principle - all buffer pairs in a homogenous solution are in equilibrium with the same hydrogen ions.
A. Bicarbonate:
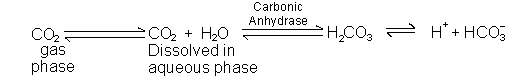
1. Open system: CO2 removed in lungs
2. CO2 dissolved in plasma (in mM/L) is equal to 0.03 x Pco2
3. CO2 + H2O![]() H2CO3; but in equilibrium, so both dissolved CO2 and H2CO3 are considered part of [HA].
The ratio of dissolved CO2 to carbonic acid is about 1,000 to 1.
H2CO3; but in equilibrium, so both dissolved CO2 and H2CO3 are considered part of [HA].
The ratio of dissolved CO2 to carbonic acid is about 1,000 to 1.
4. Note that "total CO2" = dissolved CO2 + H2CO3 + HCO-3
5. Henderson-Hasselbalch equation:
pH = pK' + log ![]()
But the H2CO-3 is negligible,
so:pH = pK' + log![]()
The pK' of this system at physiological pH's and at 38°C is 6.1.
Therefore, at pHa of 7.40 and PaCO2 of 40 torr:
7.40 = 6.1 + log ![]()
6. The [HCO-3] is therefore normally about 24 mM/L because the log of 20 is about 1.3.
7. The buffer value of plasma in the presence of Hb is 4-5 times that of plasma separated from erythrocytes. (Buffer value = H+'s in mEq/L that can be added to or removed from a solution with a resultant change of one pH unit).
B. Phosphate:
![]()
![]()
![]() ; pK is about 6.8. Other organic phosphates have pK's near 7.0: Glucose - 1-P, ATP,
etc.
; pK is about 6.8. Other organic phosphates have pK's near 7.0: Glucose - 1-P, ATP,
etc.
C. Proteins
Imidazole groups of histidine residues have pK's ranging from 5.5 to 8.5
1. Main protein: Hb. DeoxyHb is a weaker acid than is oxyHb. This allows CO2 loading in the tissues as deoxyHb can accept H+'s from the dissociation of H2CO3 and from carbamino compounds (see Levitzky, Chapter 7); and unloading in the lungs.
2. Other plasma proteins may also act as buffers.
D. Buffers of the interstitial fluid:
1. Mainly bicarbonate; some phosphate.
2. Note that the volume of interstitial fluid is much larger than that of the plasma, so the ISF may play an important role in buffering.
E. Bone:
Much calcium and phosphate in bone. Chronic acidosis may lead to bone demineralization.
F. Intracellular buffering: Intracellular proteins and organic phosphates. Note that Hb is an intracellular buffer.
V. Acidosis and Alkalosis: use of the Davenport Diagram (Levitzky Fig. 8-1)
A. Respiratory acidosis:
![]()
![]()
![]() pHa
pHa
Causes: Impairment of ![]() A (Levitzky Table 8-1)
A (Levitzky Table 8-1)
B. Respiratory Alkalosis:
![]()
![]()
![]() pHa
pHa
Causes: Hyperventilation (Levitzky Table 8-2)
C. Metabolic Acidosis
![]()
![]()
![]() pHa
pHa
Causes: ingestion, infusion or production of fixed acids; decreased renal H+excretion; loss of HCO-3 etc. e.g. diarrhea, diabetic ketoacidosis, lactic acidosis (Levitzky Table 8-3)
D. Metabolic Alkalosis
![]()
![]()
![]() pHa
pHa
Causes: Loss of fixed acids; ingestion, infusion, or excessive reabsorption of bases. e.g. vomiting; excess antacids (Levitzky Table 8-4)
VI. Compensatory Mechanisms
A. Respiratory: H+'s stimulate arterial chemoreceptors; compensation takes minutes
B. Renal: excretion of fixed acids; reabsorption of base; compensation takes hours or days. Mechanisms:
1. Secretion of H+ by tubular cells into lumen (inverse relationship with K+secretion) and reabsorption of filtered HCO-3. 90% of all HCO-3 is "reabsorbed" either directly or by carbonic acid dissociation in the proximal tubule.
2. Phosphate
3. Ammonia
4. In alkalosis the kidney decreases H+ secretion and decreases HCO-3 reabsorption. Kidney tends to reabsorb almost all filtered HCO-3 until [HCO-3]p reaches about 27-28 mEq/L.
C. Summary of Acid-Base Disorders (Levitzky Table 8-5):
pHa = a constant +![]()
VII. Base excess (or Deficit): The number of mEq of acid or base needed to titrate one liter of blood to a pHa of 7.40 at 37°C, if the Paco2 were held constant at 40 torr.
VIII. Anion gap = [Na+] - ([Cl-] + [HCO-3]); should be 12+ 4 mEq/L High anion gap (>16 mEq/L ) in metabolic acidosis suggests presence of anions that are not usually measured. Causes of high Anion Gap: (Levitzky Table 8-6)
IX. Disorders that can cause of tissue hypoxia (Levitzky Table 8-7)
Copyright 2000 M. G. LEVITZKY
This page was last updated on Thursday July 25, 2013 at 2:54 PM
LSUHSC is an equal opportunity educator and employer.
The statements found on this page are for informational purposes only. While every effort is made to ensure that this information is up-to-date and accurate, for official information please consult a printed University publication.
The views and opinions expressed in this page are strictly those of the page author. The contents of this page are not reviewed or approved by LSUHSC.
This page is maintained by webmaster-arc: mgiaim@lsuhsc.edu
