Treatment - Epilepsy Brain Implants
Responsive Neurostimulator (RNS) System
The RNS system is a new, FDA-approved implanted device designed to control seizures. The RNS is manufactured by NeuroPace, Inc. and was developed to detect and respond to seizure activity. The RNS contains a small battery for power (changed every 3-5 years) and a microprocessor (computer chip) that detects and stores electrical activity from the brain. When the RNS detects a seizure, it responds by delivering electrical stimulation through the lead(s) (electrodes) to a small part of the brain to try to stop seizures. This type of therapy is known as responsive stimulation. The device is implanted through open brain surgery and the battery is changed through a simple scalp incision. Risks and recovery time are similar to standard epilepsy surgery (resection). Physicians communicate with the device after it is implanted by using a laptop computer, called the Programmer, and a “wand”. The ongoing EEG, and especially the seizure events, are stored by the device and downloaded via the wand to the laptop. Device settings are adjusted using the same tools. LSUHSC-NO Epilepsy Center is a registered treatment site for the RNS system.
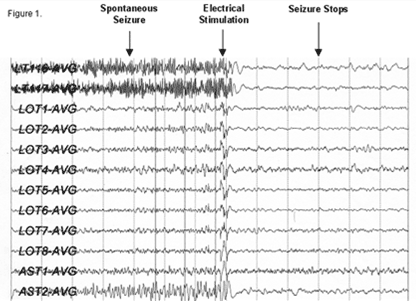
Figure 1: EEG recorded from the RNS device showing the termination
of a seizure after stimulation. (Reference: www.neuropace.com)

RNS Device: Actual size
of stimulator is 4.15 cm (w)
x 6.00 cm (l) x 0.838 cm (d
