Answer and Discussion
FISH for AML was performed for this patient and the results are as follows:
The result of FISH, along with the morphological findings result in a diagnosis of Acute Myeloid Leukemia (AML) with KMT2A rearrangement for the patient.
AML with KMT2A rearrangement is a subtype of AML which involves fusion of the KMT2A gene on chromosome 11q23.3 with a different partner gene. This entity comprises approximately 9-12% of all AML cases in children and about 2% of adult AML cases. Extramedullary involvement frequently occurs. Patients may present with gingival hypertrophy and bleeding.
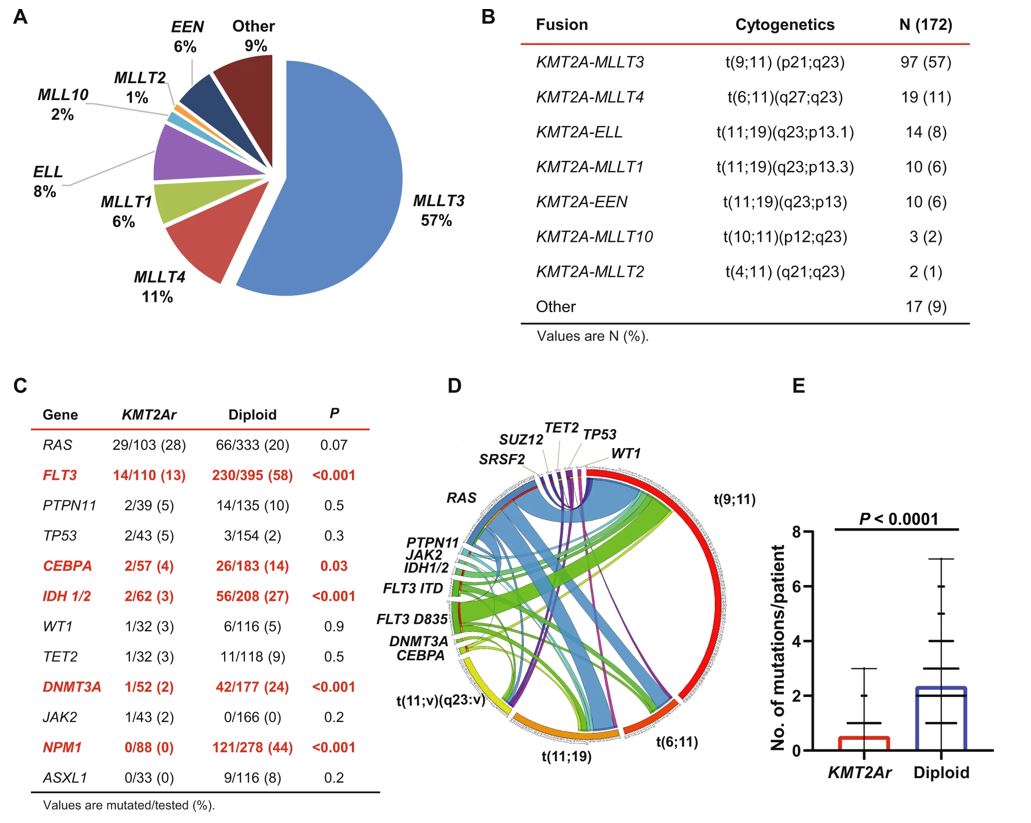
In KMT2A-rearranged AMLs more than 80 fusion partner genes have been described. Acute myeloid leukemia with KMT2A rearrangement is associated with a relatively low mutation burden compared to the AMLs with a normal karyotype. The following figure highlights distribution of fusion partner genes, associated cytogenetics and the gene mutations described in this entity.
A. Distribution of fusion partner genes. B. Cytogenetics and distribution of 11q23 translocations. C. Genes most commonly mutated in KMT2Ar AML compared to an age-matched cohort of AML with a diploid karyotype. D. Circos plot depicting patterns of co-occurrence between mutations and various translocations leading to KMT2Ar. E. Number of mutations per patient comparing KMT2Ar AML to an age-matched cohort of AML with a diploid karyotype.
Image source: Blood Cancer Journal (Blood Cancer J.) ISSN 2044-5385 (online) [Reference 3]
Histologically, AML with KMT2A rearrangement can display a wide range of morphologies, with the most common being monocytic, monoblastic, or myelomonocytic. The blast cells in these cases are generally large with basophilic cytoplasm and may contain azurophilic granules and cytoplasmic vacuolization, particularly in cases with monoblastic morphology.
The immunohistochemical profile of this AML varies with the respective lineage. Because monocytic lineage is the most common, this entity most often expresses CD33, CD4, CD65, HLA-DR, CD15, and lysozyme. Expression of CD7 is rare. Heterogenous expression of CSGP4 is common but is not required for diagnosis. CD34 and CD117, markers of immaturity, are usually negative in the monocytic lineage but can be positive in other lineages.
For diagnosis, the demonstration of the KMT2A rearrangement is essential and must be confirmed by cytogenetic/molecular studies. According to the WHO classification, increased myeloid blasts in the bone marrow or peripheral blood are needed, but a blast percentage >20% is not required for diagnosis.
AML with KMT2A rearrangement is associated with an adverse prognosis with a high risk of relapse. Treatment options include combination chemotherapy including cytarabine and idarubicin with or without a second nucleoside analog (cladribine, fludarabine, or clofarabine) and hypomethylating agents such as 5-azacitidine or decitabine. Early mortality is usually caused by development of bleeding and coagulopathy.
Differential Diagnosis:
Acute myeloid leukemia with NPM1 mutation: This entity is associated with somatic mutations involving the nucleophosmin 1 (NPM1) gene. It is often associated with leukocytosis and will often result in a higher platelet count than other AML subtypes. In contrast to AML with KMT2A rearrangement, this entity is more common in adults (occurring in 30% of adult AML patients) and has a female predominance. Morphologically, this entity is associated with a “cup-like” appearance of the nuclei of blasts, which if found in >10% of blasts is a finding highly specific for AML with NPM1 mutation. Similar to KMT2A rearrangement-associated AML, the most common morphological variants are monocytic and myelomonocytic. Like AML with KMT2A rearrangement, in presence of NPM1 mutation a blast percentage of 20% is not required for diagnosis of this entity.
Acute monocytic leukemia: Acute monocytic leukemia is an acute myeloid leukemia (AML) that lacks defining genetic abnormalities. This entity affects all age groups and predominantly involves the bone marrow and blood. Like AML with KMT2A rearrangement, extramedullary involvement is common. In patients with this entity, a bone marrow biopsy will display widespread infiltration by immature cells with high N:C ratios, distinct nucleoli, and irregularly lobulated nuclear contours. Monoblastic morphology may be present and includes intensely basophilic cytoplasm, sometimes with azurophilic granules and vacuolization. Immunophenotyping for this entity will display myeloid markers on the blasts (CD13, CD33, CD15, and CD117) as well as CD34 and HLA-DR. Markers of monocytic differentiation will also be expressed (including CD4 [dim], CD11b, CD14, CD36, and CD64). Unlike AML with KMT2A rearrangement, CD7 and CD56 are frequently expressed. Acute monoblastic leukemia is a variation of this entity characterized by infiltration by >80% blasts. Unlike AML with KMT2A rearrangement, >20% blasts or blast equivalents is required to make this diagnosis in addition to a lack of a defining genetic abnormality associated with AML.
Acute promyelocytic leukemia – microgranular subtype: Acute promyelocytic leukemia (APL or APML) is an AML characterized by predominance of promyelocytes. This entity also is defined by fusion of the PML gene with the RARA gene. Histologically, this entity is characterized by two morphological variants: hypergranular APL and microgranular APL. The microgranular subtype is characterized by blastoid cells with cytoplasm seemingly devoid of granules, resulting in an appearance that can be mistaken for myelomonocytes or monocytic morphology. Auer rods, while sparse, are often present and can help to distinguish APL from AML with KMT2A rearrangement or acute monocytic leukemia. By flow cytometry, APL promyelocytes lack expression of CD34 and HLA-DR. However, the microgranular subtype may have partial expression of both, causing diagnostic challenges. However, APL will rarely express monocytic markers (CD14 and CD64), differentiating this entity from acute monocytic leukemia. FISH for AML will definitively distinguish between APL and AML with KMT2A rearrangement if morphological evaluation is ambiguous.
References:
1. WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet;
beta version ahead of print]. Lyon (France): International Agency for Research on
Cancer; 2022 [cited 04/16/2024]. (WHO classification of Tumours Series, 5th ed.; vol.
11). Available from: https://tumourclassification.iarc.who.int/chaptercontent/63/41.
2. Chang, C. C., & Ohgami, R. S. (2017). Precision Molecular Pathology of Myeloid
Neoplasms. Springer. http://books.google.ie/books?id=H5s3DwAAQBAJ&printsec=frontcover&dq=precision+medicine+pathology+of+myeloid+neoplasms&hl=&cd=1&source=gbs_api
3. Issa, G. C., Zarka, J., Sasaki, K., Qiao, W., Pak, D., Ning, J., ... & Ravandi,
F. (2021). Predictors of outcomes in adults with acute myeloid leukemia and KMT2A
rearrangements. Blood cancer journal, 11(9), 162.
4. Falini, B., Brunetti, L., Sportoletti, P., & Martelli, M. P. (2020). NPM1-mutated
acute myeloid leukemia: from bench to bedside. Blood, The Journal of the American
Society of Hematology, 136(15), 1707-1721.
5. Dervesteanu, M., Onisai, M., Bumbea, H., Radesi, S., Ciufu, C., Nicolescu, A.,
... & Ilea, A. (2007). Acute promyelocytic leukemia microgranular variant–A clinical
and therapeutical approach. Mædica A Journal of Clinical Medicine, 2(4), 328.
6. Fournier, E., Inchiappa, L., Delattre, C., Pignon, J. M., Danicourt, F., Bemba,
M., ... & Duployez, N. (2019). Increased risk of adverse acute myeloid leukemia after
anti-CD19-targeted immunotherapies in KMT2A-rearranged acute lymphoblastic leukemia:
a case report and review of the literature. Leukemia & Lymphoma.
Board Style Questions
1. Which type of differentiation does AML with KMT2A rearrangement most commonly show?
A. Megakaryocytic
B. Erythroid
C. Monocytic
D. Lymphoid
2. Which type of prognosis is AML with KMT2A associated with?
A. No effect on prognosis
B. Good prognosis
C. Poor prognosis with a high risk of relapse