Answer and Discussion
This case represents a perivascular epithelioid cell tumor (PEComa). The tumor displays nests and sheets of tumor cells with clear to eosinophilic granular
cytoplasm, surrounded by delicate thin-walled vessels and focal spindle cell changes.
In certain areas, the tumor cells are notably arranged in a perivascular radial distribution.
The expression of melanocytic markers such as HMB45 and Melan-A, along with smooth
muscle markers including Desmin, SMA, and Caldesmon. A positive Cathepsin K further
confirms the diagnosis.
PEComas are a unique group of mesenchymal tumors that express both melanocytic and
smooth muscle markers. Gynecological PEComas comprise of just over one-fourth of the
overall PEComa. These tumors most commonly develop in the uterine corpus, and less
frequently in the cervix, vagina, ovary, and broad ligament. Patients often present
with a pelvic mass, abnormal uterus bleeding, or abdominopelvic pain. Patients typically
range from 16 to 77 years old with a mean presentation age of 51 years.
PEComas usually arise sporadically, though about 10% of cases occur associated with
tuberous sclerosis (TS). TS, an autosomal dominant genetic condition, leads to the
growth of benign tumors across various organs. A critical aspect of diagnosing TS
involves genetic testing to identify mutations in the TSC1 and TSC2 genes. TS associated
PEComas often have inactivated TSC1 or TSC2 genes, resulting in the activation of
the mTOR signaling pathway, which significantly contributes to the tumors' growth.
Some PEComas also contain gene fusions involving TFE3, RAD51B, or HTR4-ST3GAL1. The
presence of TSC gene mutations and TFE3 fusions are mutually exclusive, suggesting
distinct tumorigenic pathways. Moreover, the RAD51B-OPHN1 gene rearrangement, identified
in a limited number of PEComa cases, has been associated with recurrence and aggressive
behavior.
Uterine PEComas vary in size from 0.2 to 17.0 cm and often have well-circumscribed
margins, though some exhibit invasive growth. These tumors may have features like
hemorrhage, necrosis, or softening. Morphologically, PEComas typically consist of
nests or sheets of tumor cells surrounded by delicate thin-walled vessels, but other
structures such as trabeculae, cords, fascicles, and pseudoalveoli are also observed.
The tumors predominantly contain epithelioid cells with clear to eosinophilic granular
cytoplasm, though some also exhibit spindled cells changes, occasionally presenting
with rhabdoid or foamy appearance. Other notable features include a perivascular distribution
of tumor cells, multinucleation, and sometimes extensive stromal hyalinization.
Immunohistochemically, PEComas typically express melanocytic markers (HMB45 and Melan-A)
and smooth muscle markers (SMA, Desmin, and H-caldesmon). HMB45 is the most sensitive
of these, showing positivity in all tumors, whereas Melan-A provides more specificity
and may only be present variably. Cathepsin K expression is universal in PEComas,
significantly aiding in diagnosis. TFE3 translocation associated PEComas must be suspected
when diffuse epithelioid and clear cell morphology is seen. These PEComas are a specific
subtype and consistently exhibit diffuse positivity for TFE3, HMB45, and cathepsin
K, with weak to absent expression of smooth muscle markers. However, not all TFE3-positive
PEComas are associated with TFE3 translocations.
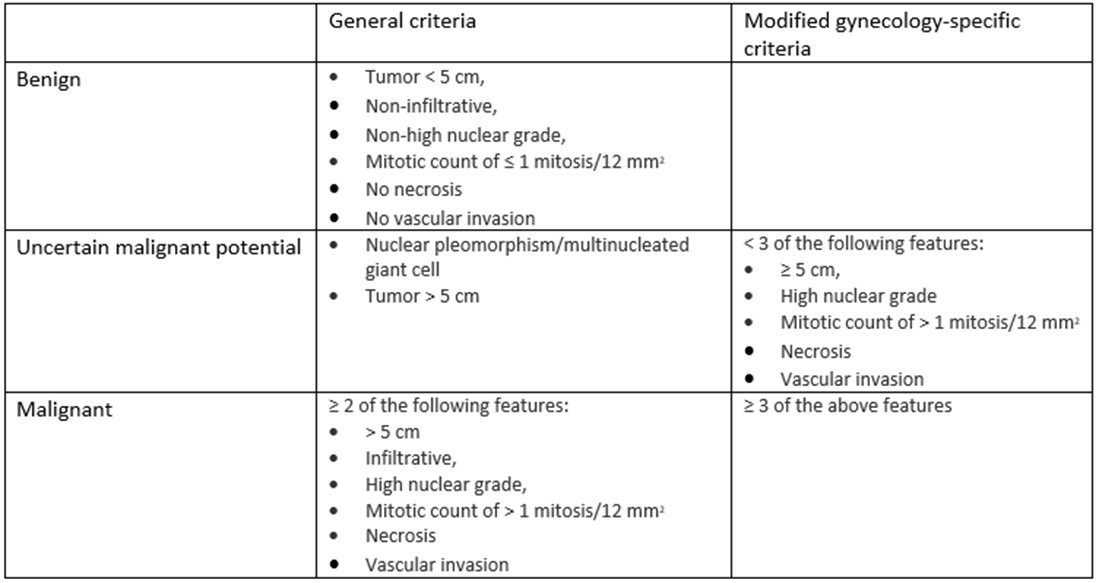
The original classification by Folpe et al. categorized PEComas as benign, of uncertain
malignant potential, or malignant. This classification was based on criteria such
as tumor size, growth pattern, nuclear grade, and other pathological features. However,
a subsequent study by Schoolmeester et al. revealed inconsistencies. The original
criteria seemed to classify benign behaving PEComas as malignant while some did not
fit under any category. In response, Schoolmeester et al proposed a gynecologic-specific
algorithm requiring at least four of five features (size ≥ 5 cm, high-grade atypia,
mitoses > 1/50 HPFs, necrosis, and lymphovascular invasion) for a malignant classification.
However, retrospectively, these criteria also led to apparent misclassifications.
Consequently, Bennett et al. recommended lowering the threshold to three atypical
features to more accurately classify aggressive PEComas (Table 1). More recently,
there has been a proposal to eliminate the "benign" category, reflecting that some
tumors can behave aggressively despite benign histology.
Table 1: Proposed algorithms for stratifying the behavior of uterine PEComas
In conclusion, PEComas are rare mesenchymal tumors known for their unique histological
and immunohistochemical features. Surgical resection remains the primary treatment
method. However, the availability of targeted therapies, such as mTOR inhibitors for
cases with TSC mutations, offers a promising avenue for enhancing patient outcomes.
A thorough understanding of the histological characteristics is crucial for distinguishing
PEComas from other similar tumors and subsequently provides effective management of
PEComas.
Differential diagnosis:
Epithelioid Smooth Muscle Tumor: These tumors are part of the broader category of leiomyomas and leiomyosarcomas and
display epithelioid cell morphology. However, unlike PEComas, they lack perivascular
arrangement and delicate thin-walled vessels, instead exhibiting large, thick-walled
blood vessels. Immunohistochemically, these tumors are negative for melanocytic markers
but may express keratin and EMA. They consistently express smooth muscle markers such
as SMA and Desmin. Genetically, leiomyomas may have MED12 mutations, HMGA1/HMGA2 rearrangements,
or FH mutations, while epithelioid leiomyosarcomas may show PGR fusions, further distinguishing
them from PEComas.
Alveolar Soft Part Sarcoma (ASPS): ASPS can be differentiated from PEComa by its distinctive alveolar structure, in
which cells are separated by fibrovascular septa. The cells in ASPS typically exhibit
a granular cytoplasm due to PAS-positive, diastase-resistant rod-shaped or rhomboid-shaped
crystals. Immunohistochemically, ASPS is negative for smooth muscle and melanocytic
markers, distinguishing it from PEComa. Additionally, the tumor characteristically
expresses the ASPSCR1:TFE3 gene fusion, which is absent in PEComa. In contrast, certain
PEComas may exhibit a PSF-TFE3 fusion, different from the fusion found in ASPS. Therefore,
both ASPS and some PEComas can exhibit positive TFE3 staining, and this marker alone
is not reliable for differentiating between these tumors. Instead, FISH testing can
be employed to identify specific gene fusions, providing a more definitive distinction
between ASPS and PEComa.
Malignant Melanoma: While melanoma can express some of the same melanocytic markers as PEComa (HMB-45
and Melan-A), it also usually expresses S100 and SOX10, which are negative in PEComa.
If there is a prior history of melanoma, this could suggest a metastatic origin. Additionally,
melanomas exhibit more pronounced cellular atypia and a more variable architectural
pattern compared to the relatively uniform appearance of PEComa cells.
Poorly Differentiated Endometrial Carcinoma: This tumor can mimic PEComa when it presents with epithelioid features. It lacks
the specific melanocytic markers seen in PEComas but can exhibit a high degree of
nuclear atypia and aggressive behavior. Immunohistochemical staining for epithelial
markers (e.g., cytokeratins and epithelial membrane antigen, EMA) and absence of melanocytic
markers help differentiate it from PEComa.
References:
1. WHO,5th Edition: https://tumourclassification.iarc.who.int/chaptercontent/34/251
2. Folpe AL, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic
origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg
Pathol. 2005 Dec;29(12):1558-75. doi: 10.1097/01.pas.0000173232.22117.37. PMID: 16327428.
3. Schoolmeester JK, et al. Perivascular epithelioid cell neoplasm (PEComa) of the
gynecologic tract: clinicopathologic and immunohistochemical characterization of 16
cases. Am J Surg Pathol. 2014 Feb;38(2):176-88. doi: 10.1097/PAS.0000000000000133.
4. Bennett JA, et al. Uterine PEComas: A Morphologic, Immunohistochemical, and Molecular
Analysis of 32 Tumors. Am J Surg Pathol. 2018 Oct;42(10):1370-1383. doi: 10.1097/PAS.0000000000001119.
PMID: 30001237; PMCID: PMC6133752.
5. Musella A, et al. Perivascular epithelioid cell neoplasm (PEComa) of the uterus:
A systematic review. Int J Surg. 2015 Jul;19:1-5. doi: 10.1016/j.ijsu.2015.05.002.
Epub 2015 May 14.
6. Cao B, Huang Y. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus.
BMC Womens Health. 2022 Dec 16;22(1):523. doi: 10.1186/s12905-022-02119-9. PMID: 36522714.
Board type review questions
1. A 48-year-old female presented to the emergency department with abdominal pain
and abnormal bleeding. Imaging studies revealed a uterine mass, and a subsequent biopsy
confirmed the diagnosis of perivascular epithelioid cell tumors (PEComas). Histological
examination showed epithelioid tumor cells with clear to eosinophilic cytoplasm and
arranged in nests architecture surrounded by delicate blood vessels. Which gene alteration
is commonly associated with diffuse clear cell/ eosinophilic morphology in PEComas?
A. TSC1/2 mutation
B. P53 mutation
C. RAD51B-OPHN1 fusion
D. TFE3 fusion
E. HTR4-ST3GAL1 fusion
2. A 56-year-old female presented to the hospital with a myometrial mass and liver
metastases. After undergoing a hysterectomy, she was diagnosed with perivascular epithelioid
cell tumors (PEComas). Which gene alteration is associated with an adverse prognosis
in PEComas?
A. TSC1/2 mutation
B. P53 mutation
C. RAD51B-OPHN1 fusion
D. TFE3 fusion
E. HTR4-ST3GAL1 fusion
D. TFE3 fusion
C. RAD51B-OPHN1 fusion
