
Alberto E. Musto, M.D., Ph.D.
Alberto E. Musto, M.D., Ph.D.
Assistant Professor of Research, Neurosurgery and Neuroscience
Assistant Professor of Research, Neurosurgery and Neuroscience
2020 Gravier Street,
9th Floor, Suite D
Office: 946
New Orleans, LA 70112
Office Phone: (504) 599-0846
Fax: (504) 599-0488
amusto@lsuhsc.edu
Bio
Degrees
-
Bachelor in Commercial Sciences, School of Commerce “Carlos Pellegrini”,
Universidad de Buenos Aires (UBA), Argentina (AR) 1985. -
Medical Doctor (M.D.), School of Medicine, UBA. AR 1991.
-
Doctor of Philosophy(Ph.D. in Neurobiology),School of Medicine, UBA, AR 2002.
-
Board Certified Neurologist, School of Medicine, UBA, AR 2002.
-
Board Certified Radiologist, National Ministry of Public Health,AR 2002.
Post-Graduate Education
-
Postgraduate Studies in Academic Education, School of Medicine, UBA, AR 2000.
-
Master in Hospital Management and Health Systems.
Centro de Altos Estudios en Ciencias Exactas (CAECE) AR
(Thesis dissertation pending http://www.ucaece.edu.ar/index.php/ceas-area-de-investigacion/)
2002. - Postdoctoral Research Fellow. Neuroscience Center of Excellence.
Louisiana State University Health Science Center, USA. (2002-2006).
Awards/Recognitions
- 1990 Award: “Estímulo al Futuro en Neurociencias”, for the best scientific work in neurosciences.
Semana de Congresos del Sistema Nervioso (SEMCOSIN), Annual Meeting of Sociedad Argentina de Neurociencias. AR.
- 1994-1998 Competitive Individual Fellowship Award from the Council of Scientific and Technological Research of Argentina (CONICET)
to complete graduate studies in Development and Regeneration of the nervous system. AR.
- 1992 Fellow in Neuroradiology, Fundación para el Estudio del Sistema Nervioso (FESIN), Clinica del Sol. AR.
- 1995 Scholarship to assist to the 23rd Course: Neurobiology, North Atlantic Treaty Organization (NATO), Advanced Study Institute:
International School of Biophysics "Antonio Borsellino", Ettore Majorana International Centre for Scientific Culture. Erice, Sicily, Italy.
- 1999 Group Study Exchange Scholarship.Rotary Foundation-Rotary International. University of
Rochester-Medical Center and Cornell University, New York State, USA
. - 2005 Paul Harris Fellow. Rotary Foundation, Rotary International.
- 2010 National Center for Research Resources Travel Award. National Institute of Health.
- 2012 Global Alumni Service Humanity Award. Winner Zone 22 B&C. Rotary International.
- 2012 LSU Neuroscience Center of Excellence. Awarded for outstanding research contribution.
Editorial Activities
Journal Editorial Board:
- Modern Research in Inflammation (2012-present).
- Journal of Life Medicine (2012-present).
- Austin Journal of Clinical Neurology (2013-present).
Manuscript Reviewer:
- Journal of Pediatric Neurology (2007-present).
- Epilepsy Research (2010-present).
- Neuronal Regeneration Research (2012-present).
-
Journal of Neurological Sciences (2014-present).
Clinical Interests
Clinical Research Experience
-
Guidelines for Epilepsy Surgery- Guias para la Cirugia de la Epilepsia. Bernater; F. Bruno, V. Campanille, E. Centurikon, D. Consalvo, A Cruz, E Ferraro, A. Firstenfedl E. Olanidni, B. Gogante, R. Granillo, j. Grippo, S. Kochen, E. Lekuniec, D. Lopez, E. Mallo, C.Marquez Vigo, Mesri, A. Musto, Perasolo, P. Saidon, W. Silva, C Tabeada, A Thompson, J. Ure and M.Viaggio. Rev. Neurol. Argentina 2006; 31: 117-122
-
Las Epilepsias en un Hosptial Publico: Experiencia Enero 1994 -Junio 1997. Servicio de Neurología del Hospital Municipal de San Isidro): Granillo Rubens, Musto Alberto, Matera Raúl, Mauriño Alberto, Bettinelli Ricardo. http://www.drwebsa.com.ar/matera/ephp.htm
Research Interests
Epilepsy is a neurological disease characterized by spontaneous recurrent seizures. A seizure is a manifestation of abrupt episodes of neurological dysfunction associated with abnormal discharges of neuronal networks. Clinical outcome of seizures depend on the region affected by those discharges. Epilepsy affects 65 million people worldwide. Among the different types of epilepsies, temporal lobe epilepsy (TLE) or limbic epilepsy is the most common form, which has no cure, no effective treatment, no biomarker/s for its early diagnosis, and no preventive therapies. Certain brain injuries induce the subsequent development of TLE. The primary need and most urgent issue in epilepsy research is to determine the fundamental neurobiological mechanism underlying the development of TLE, which has been termed limbic epileptogenesis.
The overall goals of my research are:
- To understand the pathophysiology of limbic epileptogenesis.
- To identify biomarkers for its early diagnosis.
- To provide a new experimental approach that contributes to its cure with potential application to other neurological diseases related with seizures.
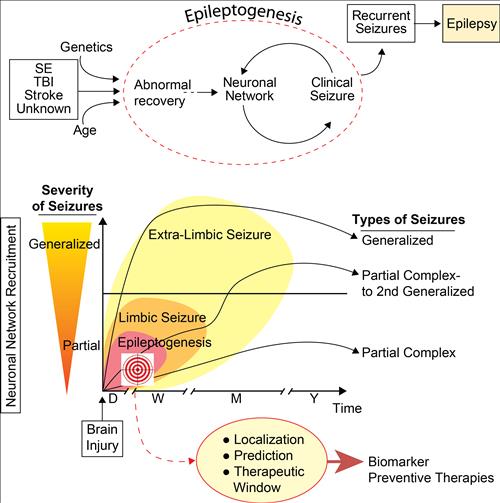 Figure 1:
Concept of epileptogenesis focusing on our studies (target) in early stages of epileptogenesis in order to localize, predict and provide therapeutics for diagnostic and prevention of epileptogenesis.
Figure 1:
Concept of epileptogenesis focusing on our studies (target) in early stages of epileptogenesis in order to localize, predict and provide therapeutics for diagnostic and prevention of epileptogenesis.
Current Research:
Limbic Epileptogenesis, Neuronal Circuits and Neuroinflammation :
I am studying the hippocampal neuronal circuitry activity in freely-moving rodents from different models of epilepsy. I have observed electrical oscillations such as theta, gamma and high frequency oscillations, ripple-sharp wave complex or sharp waves, recorded from chronically-implanted microelectrodes (silicon probes) in the hippocampus. These electrical activities are associated with determined behavior, such as locomotion, memory or sleep, and they arise from an organized neuronal network of synchronized interactions of pyramidal and inter-neuronal cell activity responsible for the physiological balance of excitatory and inhibitory forces in the brain. Disruption of this balance results in neuronal hyper-excitability and seizures.
Using experimental models of epileptogenesis, I found that there is an increase in episodes of altered behavior associated with the disruption of physiological electrical patterns in hippocampal neuronal circuitry, including spontaneous micro-epileptiform activities and neuronal hyper-excitability, thus suggesting the formation of aberrant circuitry activity many days before severe spontaneous recurrent seizures occur.
Interestingly, when platelet-activating factor (PAF) receptor antagonist or a potent neuroprotective docosahexaenoic acid (DHA)-derived lipid mediator, neuroprotectin D1 (NPD1), are administered systemically in epileptogenesis, neuronal hyper-excitability, seizure susceptibility and neuronal damage are attenuated, suggesting that an imbalance in inflammation resolution, also called neuroinflammation, could be responsible for the development of neuronal epileptic circuit.
To gain a better understanding of the structural modification of hippocampal neuronal circuitry in epileptogenesis, I have focused on the dendritic spines of the pyramidal neurons of CA1 and granular cells of the dentate gyrus. The dendritic spines represent the cellular site of synapsis, receive and integrate excitatory (glutamaergic) synaptic inputs that meditate neuronal excitability, and are implicated in the mechanism of synaptic plasticity and learning. The dendritic spines are damaged after hyper-synchronous electrical activity in the absence of neuronal death or clinical seizures. Also, they present abnormalities in Alzheimer’s disease, and genetic and mental disorders, with a high prevalence of seizures.
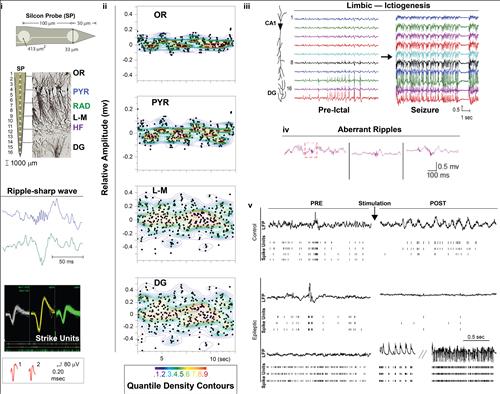 Figure
2a,
i: Approach to study hippocampal neuronal activity in vivo using silicon probe for local field potential spike units recordings in experimental models of epilepsy, ii: Representative average of hippocampal local field potential; iii: progression of epileptic discharge in hippocampal circuitry; iv aberrant ripples in ictiogenesis; v: neuronal hyper-excitability in epilepsy.
Figure
2a,
i: Approach to study hippocampal neuronal activity in vivo using silicon probe for local field potential spike units recordings in experimental models of epilepsy, ii: Representative average of hippocampal local field potential; iii: progression of epileptic discharge in hippocampal circuitry; iv aberrant ripples in ictiogenesis; v: neuronal hyper-excitability in epilepsy.
The aims of this research are:
- To determine the neurotransmission mechanism responsible for micro-epileptiform activities in epileptogenesis.
- To characterize the cyto-architecture of dendritic spines that contributes to the formation of aberrant circuitry in epileptogenesis.
- To elucidate key inflammatory molecules and their cellular signaling in dendritic spines that mediate aberrant neuronal network activity.
- To evaluate antiepileptogenic effects of novel neuroprotective and anti-inflammatory compounds.
- To develop innovative pre-clinical approaches in order to improve anti-epileptic drug development and clinical research in epilepsy.
- By translating the laboratory results into new diagnostic tools and treatments, this research propose to localize the epileptic circuitry, predict clinical epilepsy and identify new targets for anti-epileptogenic therapeutics.
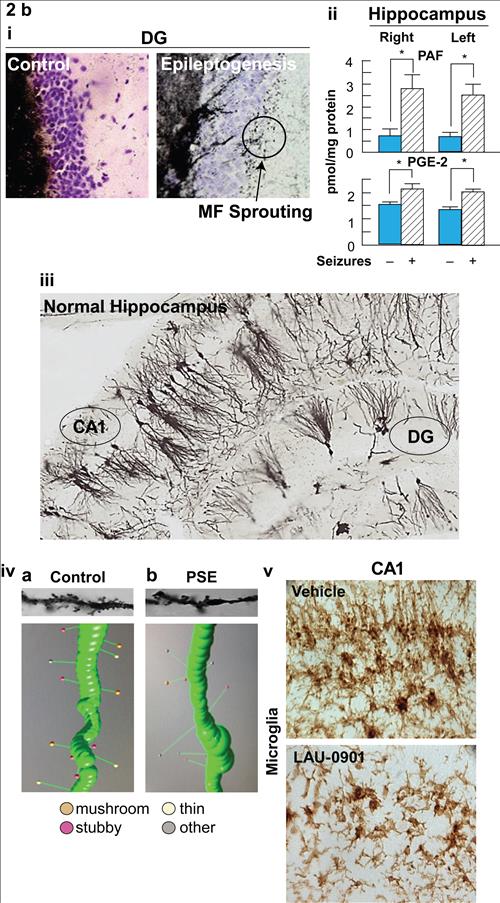 Figure 2b,
Cellular and molecular targets of epileptogenesis. i: mossy fiber (MF) sprouting in epileptogenesis; ii: platelet activating factor (PAF) and prostaglandin E2 (PGE2); iii: dendrite organization of hippocampus; iv: dendritic spines; v: microglial cells.
Figure 2b,
Cellular and molecular targets of epileptogenesis. i: mossy fiber (MF) sprouting in epileptogenesis; ii: platelet activating factor (PAF) and prostaglandin E2 (PGE2); iii: dendrite organization of hippocampus; iv: dendritic spines; v: microglial cells.
Gliobalstoma Multiforme and Neuronal Networks
Gliobalstoma Multiforme (GBM), a malignant and one of the most aggressive brain tumors, triggers seizures and contributes to increased morbidity and mortality in GBM patients. The overall goal is to identify new experimental treatments not only to prevent tumor growth but also to protect peri-tumoral neuronal neuronal activity. Using an experimental model of GBM, we are evaluating the cellular and molecular mechanism by which GBM alters the peritumoral neuronal networks responsible of neuronal dysfunction, such as hyper-excitability and seizure susceptibility. These outcomes could be translated to in more effective therapies that would improve survival rates and quality of life for brain tumor patients.
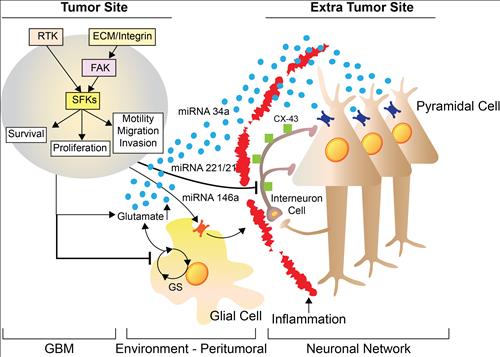 Figure 3a, Proposed cellular and molecular mechanism of GBM-induced seizures. RTK: receptor tyrosine ki
nase; ECM: extra-cellular matrix; FAK: Focal Adhession Kinase, SFs: Src family kinases; GS: Glutamine synthetase; CX-43: connexin 43.
Figure 3a, Proposed cellular and molecular mechanism of GBM-induced seizures. RTK: receptor tyrosine ki
nase; ECM: extra-cellular matrix; FAK: Focal Adhession Kinase, SFs: Src family kinases; GS: Glutamine synthetase; CX-43: connexin 43.
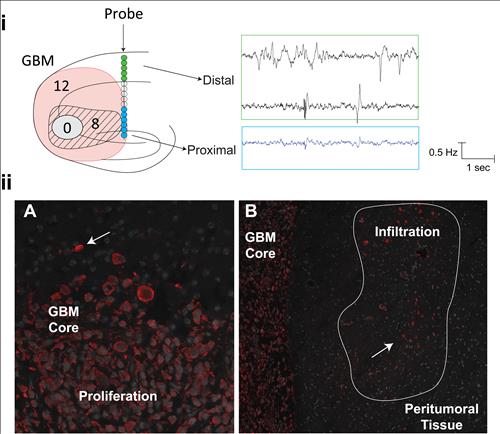
F
igure 3b (Below), i:S
chematic representation of xenograph implantation in dorsal hippocampal region simultaneously with silicon insertion ipsilateral to the GBM. Right: spontaneous LFP recordings. ii: GBM and peritumoral neuronal tissue from mouse brain.
 Acquired aberrant neuronal oscillations:
Acquired aberrant neuronal oscillations:
In addition, we observed that, in in vivo models of stroke and Alzheimer’s disease, the brain elicits abnormal neuronal network activities similar to those in animals undergoing epileptogenesis, sug-gesting that there is a temporal dynamic pattern of aberrant neuronal network following brain injuries that could indicate development of certain neurological deficit or cognitive impairment before the onset of severed clinical signs. Ultimately, this research could help to understand how particular electro-physiological signatures facilitate the diagnostic of diseases such as autism and Alzheimer’s disease or schizophrenia, and contribute to the development of pharmacological or electrical interventions that can attenuate cognitive dysfunction or enhance cognition.
Experimental Approach:
I am applying behavioral procedures, state-of-the-art in vivo electrophysiology, {MAP system, stimulator Plexon with capability to record simultaneously 64 channels using silicon probes Neuronexus}, immunohistological techniques, biochemical protocols, and novel chemical compounds against epileptogenesis and tumorogenesis in in vivo animal models of epilepsy and GBM. This research could provide reliable biomarkers for epileptogenesis and its co-morbidities, especially after brain injury, thus resulting in new methods for predicting and/or preventing seizures. Ultimately, this research could translate into new treatments for patients with epilepsy or seizure disorders in diseases such as autism and Alzheimer’s disease, or stroke and brain tumors.
Key words:
Epileptogenesis, Epilepsy, Seizures, Neuroinflammation, PAF, Dendritic Spines, Neuronal Network, Brain Tumors, DHA, Hippocampal Circuitry, Synaptic Plasticity, Oscillations, Ripples, Glioblastoma Multiforme.
Teaching Activities
Mentorship in Research:
-
Research Experiences for Underrepresented Minority Undergraduates in Basic and Genetic Research at Louisiana State University Health Sciences Center (LSUHSC). National Sciences Foundation. PI: F. Tsien, Co-PI: AE. Musto, H. Farris. Link: http://www.medschool.lsuhsc.edu/genetics/reu.aspx
-
Research Mentorship for the Fellowship Program on Hematology-Oncology, Department of Pediatric, Children’s Hospital New Orleans. Project: Peritumoral Protection of Neuronal Network by DHA. Fellow Investigator: C.Raulji, M.D (Children’s Hospital, LSUHSC). Mentor: AE. Musto and N.G. Bazan.
-
Supervised Undergraduate Research Experiences (SURE). EPSCoR-Louisiana State University, Board of Regents. Project: Synchronization of Local Field Potential in the Hippocampus. PD: Musto, AE. Student: CP Walker (Xavier University).
-
Summer Undergraduate Program in Neurosciences (SUN) LSUHSC-Neuroscience Center (SUN pIctures) and Louisiana Biomedical Research Network (LBRN) LSU and link http://lbrn.lsu.edu/summer-research/
Selected Lectures, Seminars and Presentations:
LSUHSC-School of Medicine
-
Epilepsy, Investigative Neuroscience Course (Neuro 203), topic: LSUHSC-Neuroscience Center, New Orleans (2007-present).
-
Defining Epileptogenesis for Neuroprevention. Department of Physiology, School of Medicine. LSUHSC, New Orleans, USA, (2012).
-
Glioma and Seizures. Department of Biochemistry, School of Medicine. LSUHSC, New Orleans, LA, USA, (2012).
-
Brainstem Syndromes, Clinical Case Studies in Neurosciences. LSUHSC School of, (2014-present).
-
Brainstem, Anatomy Laboratory. LSUHSC School of Medicine, (2014-present).
- Neurological Examiner, LSUHSC, School of Medicine, (2014-present).
Invited
National :
-
Impairment of Hippocampal Oscillatory Activity In Early Epileptogenesis. South East IDeA regional meeting, Charleston, SC. USA (2009).
-
Targeting Inflammation in Epileptogenesis, Tulane University, Neuroscience Program, New Orleans, LA. USA (2010).
-
Hippocampal microseizures in epileptogenesis, American Epilepsy Society, San Antonio, TX. USA, (2010).
-
A Journey to Understanding the Brain, LBRN program, New Orleans, LA, (2013).
- Neuroinflammation in Epileptogenesis, Southern University, New Orleans, LA, (2014).
International:
-
Disruption of Neuronal Network in Epileptogenesis
. International Forum on Neuroscience- 7th International
Stroke Summit, Nanjing, Jiangsu, China, (2011).
Current Lab Members:
Former Lab Members
Musto Lab 2013
Musto Lab 2012
Musto Lab 2011
Musto Lab 2010
Musto Lab 2008
Committees & Administrative Responsibilities
Study Sections
-
Member, Alzheimer Association International Research Program (2012-present).
-
Member (ad hoc), National Institute of Health (NIH), Development Brain Disorders Study Section. Center for Scientific Review.(2012).
-
Member (ad hoc), National Institute of Health (NIH), Counter Measures Against Chemical Threats Panel Reviewer (2013).
-
Member (ad hoc), LSUHSC-New Orleans, Alcohol and Abuse Center of Excellence. Reviewer (2013)
-
Chair group member, Student & Resident Education Workgroup- American Epilepsy Society (2013,2014) /www.aesnet.org/professional_education/epilepsy_education_programs
Selected Publications
Peer-Reviewed Journals
-
Marrero, L., Wyczechowska, L., Musto, A.E., Zapata, A.,Vashista, H., Parsons, C.,Wieland, S., Levitt, D., Reiss, K., Prakash, O.. Therapeutic efficacy of Aldoxorubicin in an intracranial xenograft mouse model of human glioblastoma. Neoplasia, 2014 (In press).
-
Musto, A.E, Walker, C.P., Raulji, C., Bazan, N.G. PAF antagonism counteracts epileptic hippocampal neuronal network impairments. 2014 (Under revision).
-
Musto, A.E., Walker, C.P., and Bazan, N.G. Hippocampal Neuro-Networks and Dendritic Spine Perturbations in Epileptogenesis
Are Attenuated by Neuroprotectin D1 Plos One, 2015. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0116543 -
Prakash, O., Lukiw,W.J., Reiss, K., Peruzzi, F., Musto, A.E. Gliomas and epileptic seizure. Medical Hypothesis, 2012, 2012, 79; (5) 622-6.
-
Addae, C., Yi, X., Gernapudi, R., Cheng, H., Musto, A.E., Martinez-Ceballos, E. All -Trans-Retinoid Acid Induces the Differentiation of Encapsulated Mouse Embryonic Stem Cells into GABAergic Neurons. Differentiation, 2012, 83, 233-241. PMID: 22466603.
-
Sfondouris, J.,Quebedeaux, T., Hodlgraf, C., Musto, A.E. Combined process automation for large-scale EEG analysis. Computers in Medicine and Biology, 2012, 42 129-134. PMID: 22136696.
-
Bazan N.G., Musto, A.E., Knott, E.J. Endogenous Signaling by Omega-3 Docosahexaenoic Acid-derived Mediators Sustains Homeostatic Synaptic and Circuitry Integrity. Mol. Neurolobiol. 2011 44 (2): 216-22: PMID: 21918832.
-
Musto, A.E., Samii, M. Platelet activating factor-receptor antagonism targets neuroinflammation in epilepsy. Epilepsia, 2011, 52, 551-61. PMID: 21204830.
-
Musto, A.E., Gjorstrup, P., Bazan, N.G. The omega 3 fatty acid derivative NPD1 attenuates hippocampal hyperexcitability seizure susceptibility in kindling epileptogenesis. Epilepsia, 2011, 52(9): 1601-8. PMID: 21569016.
-
Musto, A.E., Samii, M., Hayes, J.F. Different phases of afterdischarge during rapid kindling procedure in mice. Epilepsy Res. 2009, 85: 199-205. PMDI 19375287.
-
Bazan N.G., Musto, A.E. Inositol lipid signaling in synaptic activity, neuronal plasticity and epileptogenesis. Review in Encyclopedia of Basic Research in Epilepsy, Elsevier, 2009 P. Schwartzkroin (Editor).
-
Cole-Edwards K.K, Musto, A.E., Bazan, N.G. C-Jun N-terminal kinase activation responses induced by hippocampal kindling are mediated by reactive astrocytes. J Neurosci. 2006, 26 (32): 8295-304. PMID: 16899724.
-
Musto A.E., Bazan, N.G. Diacylglycerol kinase epsilon modulates rapid kindling epileptogenesis. Epilepsia. 2006, 47 (2): 267-76. PMID: 16499750.
-
Lukiw, W.J., Cui, J.G., Musto, A.E., Musto, B.C., Bazan, N.G. Epileptogenesis in diacylglycerol kinase epsilon deficiency up-regulates COX-2 and tyrosine hydroxylase in hippocampus. Biochem. Biophys. Res. Commun. 2005,338 (1): 77-81. PMID: 16137646.
-
Musto A.E., M. Hardy, Bazan N.G. Arachidonoyl-Inositol Lipid Signaling is required for Hippocampal Excitability in Kindling Epileptogenesis. Neurobiology of Lipids, 2004,Vol. 3, 4.
-
Mc Dermott C.M., LaHoste, G.J., Chen, C., Musto, A.E., Bazan, N.G., Magee, J.C. Sleep deprivation causes behavioral, synaptic and membrane excitability alterations in hippocampal neurons. J. Neurosci. 2003, 22; 23 (29): 9687-95. PMID: 14573548.
-
Marcheselli V.L., Hong, S., Lukiw, W.J., Tian, X.H., Gronert, K., Musto, A.E.,Hardy, M., Gimenez, J.M., Chiang, N., Serhan, C.N., Bazan, N.G. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem., 2003, 31; 278 (44): 43807-17. Erratum in: J Biol Chem. 2003 278 (51): 51974. PMID: 12923200.
Selected Abstracts: -
Therapeutic efficacy of a novel anthracycline derivative aldoxorubicin in an orthotopic xenograft model of human glioblastoma. Prakash, O., Musto,A.E., Wyczechowska, D., Marrero, L., Zapata, A., Walker, C.P., Parsons, C., Wieland, S., Levitt, D., Reiss, K. LCRC meeting, New Orleans 2014.
-
Prakash, O., A.E. Musto, Wyczechowska, D., Marrero, L.,Zapata, A., Vashistha, H.,Parsons, C., Wieland, S.,Levitt, D., and Reiss, K.. Preclinical evaluation of the novel anthracycline derivative aldoxorubicin for human glioblastoma chemotherapy. ESMO, Amsterdam, Netherlands, 2013.
-
Musto, A.E., Walker, C.P., and Bazan, N.G. Platelet activating factor antagonism attenuates epileptogenesis.. 5th International Conference on Phospholipase A2 Mediated Signaling in Translational Medicine, New Orleans, LA 2013.
- Musto, A.E., Walker, C.P., Bazan,N.G. Neuroprotectin D1 Attenuates aberrant neuronal networks in epileptogenesis. 5th International Conference on Phospholipase A2 Mediated Signaling in Translational Medicine, New Orleans, LA 2013.
-
Musto, A.E., Quebedeaux, T.M., Khoobehi, D., Walker, C.P., and Bazan, N.G.Neuroprotectin D1 attenuates epileptogenesis. Society for Neuroscience, New Orleans, LA 2012. Peer-reviewer abstract.
-
Musto, A.E., Tsien, F., Walker, C.P., and Farris, H. Neuroscience translational research program for underrepresented minority students in Louisiana. Society for Neuroscience, New Orleans, LA 2012. Peer-reviewer abstract.
Addae, C. Yi, X., Gernapudi, R., Cheng, H., Musto, A.E., and Martinez-Ceballos, E. Generation of GABAergic neurons from encapsulated mouse ES cells. FASEB, 2012.
-
Musto, A.E., Prakash, O., Bhattachrjee, B., Brandon, J., Lukiw, W., Reiss, K., Culicchia, F. Lyn as a potential target for gliomas related with epilepsy. American Epilepsy Society, San Diego, CA 2012. Peer-reviewer abstract.
-
Musto, A.E. Khoobehi, K.D., Walker, C.P., and Bazan, N.G. Neuroprotectin D1 attenuates epileptogenesis. American Academy of Neurology, New Orleans, LA 2012. Peer-reviewer abstract. The abstract was selected as one of the 5% top of the program and presented in the “Scientific Program Highlights Plenary Section”, April 2012.
-
Musto, A.E. and Bazan, N.G. Hippocampal microseizures and neuronal dysfunction as a target for anti-epileptogenesis strategies. Southeast Regional IDEA-NIH, New Orleans, LA 2011.
-
Musto, A.E. and Bazan, N.G. Neuroprotectin D1 attenuates epileptic hyperexcitability modulating DG-CA3 circuitry associated with interneuron survival. Southeast Regional IDEA-NIH New Orleans, LA 2011.
-
Musto, A.E. and Bazan, N.G. Neuroprotectin D1 induces interneuronal-parvalbumin plasticity as a compensatory mechanism in epilepsy. Southeast Regional IDEA-NIH New Orleans, LA 2011.
-
Musto, A.E, Quebedeaux,T.M.,Sfondouris, J., Bazan, N.G. The omega-3 fatty acid-derived mediator neuroprotectin D1 (NPD1) limits dentate gyrus hyperexcitablity and seizure susceptibility. American Epilepsy Society, 2011. Baltimore, MD Peer-reviewer abstract.
-
Musto, A.E, Quebedeaux,T., Sfondouris, J., Miller, E. Neuroinflammation triggers modifications in the neuronal network related with hipppocampal hyperexcitabiltiy and seizure susceptibility. Society for Neurosciences, Washington, DC 2011.
- Clement, C. Tiwari, V., Musto, A.E., Lukiw, W., Bhattacharjee, P., McFerrin, H.,Thompson, H., Hill, J. Neuroinvasiveness of HSV-1 high and low phenotypic reactivators in aged infected human knock-in homozygous ApoE4 transgenic mice. ARVO, Vancouver, Canada 2011.
-
Musto, A.E. Quebedeaux, T.M., Bazan, N.G. Hippocampal microseizures in epileptogenesis. American Epilepsy Society, San Antonio, TX 2010. Peer-reviewer abstract presented in platform session: “Translational Research”.
- Miller, E., Quebedeaux, T., and Musto, A.E.Hippocampal hyperexcitability and interneuronal-parvalbumin modifications as consequences of status epilepitcus. Travel Award for Annual National Student Research Forum, Galveston, TX 2011.
-
Musto, A.E., Quebedeaux, T.M., and Bazan, N.G. Targeting neuroinflammation in epilepsy: PAF antagonism reduces seizure susceptibility and limits somatostatin cell loss. NIH, NCRR Third Bienal National IDeA Symposium of Biomedical Research Excellence (NISBRE). Bethesda, MD 2010. Peer-reviewer abstract and highlighted poster.
-
Musto, A.E., Quebedeaux, T.M., Bazan, N.G. Neuroprotectin D1-mediated hippocampal interneuronal protection in seizure-induced damage. NIH, NCRR Third Bienal National IDeA Symposium of Biomedical Research Excellence (NISBRE). Bethesda, MD, 2010. Peer-reviewer abstract.
-
Musto, A.E., Quebedeaux, T.M., Bazan, N.G. Epileptiform activities as early markers for epileptogenesis. NIH, NCRR Third Bienal National IDeA Symposium of Biomedical Research Excellence (NISBRE). Bethesda, MD 2010. Peer-reviewer abstract.
-
Musto, A.E., Quebedeaux, T.M., Holdgraf, C., Canavier, C., and Bazan, N.G. Hippocampal epileptiform activity is counteracted by the novel mediator Neuroprotectin D1. Louisiana NCRR/IDeA 2010 Biomedical Research Symposium, Baton Rouge, LA, 2010. Peer-reviewer abstract.
- Musto, A.E. and Bazan, N.G. Platelet activating factor receptor antagonism during epileptogenesis. New Orleans. Louisiana NCRR/IDeA 2010 Biomedical Research Symposium, Baton Rouge, LA, 2010.
-
Musto, A.E. and Bazan, N.G. Neuroprotectin D1 (NPD1) induces hippocampal neuroprotection in experimental epileptogenesis. Louisiana NCRR/IDeA 2010 Biomedical Research Symposium. Baton Rouge, LA, 2010.
- Musto, A.E. Quebedeaux, T.M., Holdgraf, C., Canavier, C., and Bazan, N.G. Impairment of hippocampal oscillatory activity in early epileptogenesis Platform. Southeast NIH IDeA Regional. Charleston, SC 2009. Peer-reviewer abstract
Additional Info
Current funding
-
Research Experiences for Underrepresented Minority Undergraduates in Basic and Genetic Research at Louisiana State University Health Sciences Center (LSUHSC). National Sciences Foundation. PI: F. Tsien, Co-PI: AE. Musto, H. Farris. 2014-2017
-
Hyundai Clinical Award-Hope on Wheels program, Children’s Hospital of New Orleans, Peritumoral Protection of Neuronal Network by DHA. Department of Pediatrics-Sub-contract: LSUHSC Neuroscience Center. Project: Young Investigator: C.Raulji, M.D. Mentor: AE. Musto and NG Bazan (2014-present).
-
Center of Biomedical Research Excellence NIH. Mentoring Neuroscience in LA. A Biomedical Program to Enhance Neuroscience Grant # P20RR016816. PI: N.Bazan (2014 –present).
-
http://theadvocate.com/news/5393095-123/children-taught-about-brains
-
http://www.eurekalert.org/pub_releases/2014-04/lsuh-lan042414.php
- http://europepmc.org/articles/PMC3645884
-
LSU Health New Orleans: Novel compound switches off epilepsy development
http://www.sciencedaily.com/releases/2015/01/150128113830.htmLSU Health New Orleans research finds novel compound switches off epilepsy development
http://www.eurekalert.org/pub_releases/2015-01/lsuh-lhn012815.php#.VMmbG8hOirE.mailto

 myLSUHSC
myLSUHSC