Responsive Neurostimulator (RNS) System
 |
|
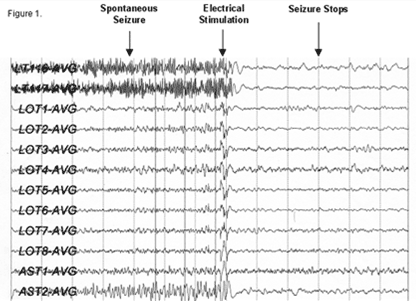
Figure 1: EEG recorded from the RNS device showing the termination |
The RNS system is a new, FDA-approved treatment to try to control seizures. The RNS is an implantable device, manufactured by NeuroPace, Inc., which detects and responds to seizure activity. The RNS contains a small battery for power (changed every 3-5 years) and a microprocessor (computer chip) that detects and stores electrical activity from the brain. When the RNS detects a seizure, it responds by delivering electrical stimulation through the lead(s) (electrodes) to a small part of the brain to try to stop seizures. This type of therapy is known as responsive stimulation and has been approved in the treatment of epilepsy.
The LSUHSC-NO Epilepsy Center is a registered treatment site for individuals who have
been implanted with the RNS system; however, we cannot perform new implants at this
time.
RNS Device: Actual size
of stimulator is 4.15 cm (w)
x 6.00 cm (l) x 0.838 cm (d)
