Welcome to the Wojcik Lab
My research group uses genetic and biophysical methods to dissect the regulation of the cytoskeleton, particularly during cell division. Currently, we are interested in kinesin mechanoenzymes, a family of molecular motors that are essential for life and that build and operate microtubule-based structures inside eukaryote cells, such as the mitotic spindle and cell division machinery.
The lab uses both genetic mutational analysis as well as pharmacologic small molecule agents to probe the biophysical basis of operation of mitotic kinesin molecular motors. We try to use an appropriate model system that is appropriate for the scientific questions we would like to answer, including the classical genetic system, Drosophila melanogaster, in vitro human cell cultures, or biophysical analysis and structural dissection.
Research
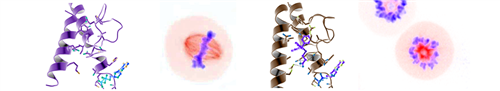
One major project in the lab centers on probing the biophysical mechanisms of kinesin behaviors on microtubules. Using a broad range of methods, ranging from classical genetic and evolutionary, to biophysical and structural approaches, we are elucidating how the design of this mechano-enzyme can be subtly tuned to either function as a molecular motor that moves along microtubule tracks, or instead behave as a polymerizer or depolymerizer of microtubules. We have chosen to study the human mitotic kinesin in the Kinesin-5 family, HsEg5, that is necessary for the biogenesis of the mitotic spindle in eukaryotic cells. One of the tools we use to study HsEg5 are small chemical allosteric inhibitors, which aid in biophysical analysis of kinesin motor function, and are, at the same time, being aggressively pursued as a new class of clinical anti-cancer therapeutics.
A second major project has been developed in the lab based on our discovery of small chemical inhibitors of a mitotic Kinesin-5 in malaria parasites, including Plasmodium falciparum and vivax (PfEg5 and PvEg5, respectively). By leveraging our work on the human homolog to this important disease-causing organism, we have been able to identify small chemical inhibitors that exhibit exquisite selectivity for the malaria Kinesin-5 homologs without any cross-reactivity to the human homologs. Current work seeks to exploit our novel inhibitors to probe mechanisms of cell division with this disease organism. Our work is proof of principle that kinesins represent novel druggable targets with built-in selectivity for small chemical inhibitors and a potential source of badly needed new anti-malarial compounds.
